Scientific Abstract
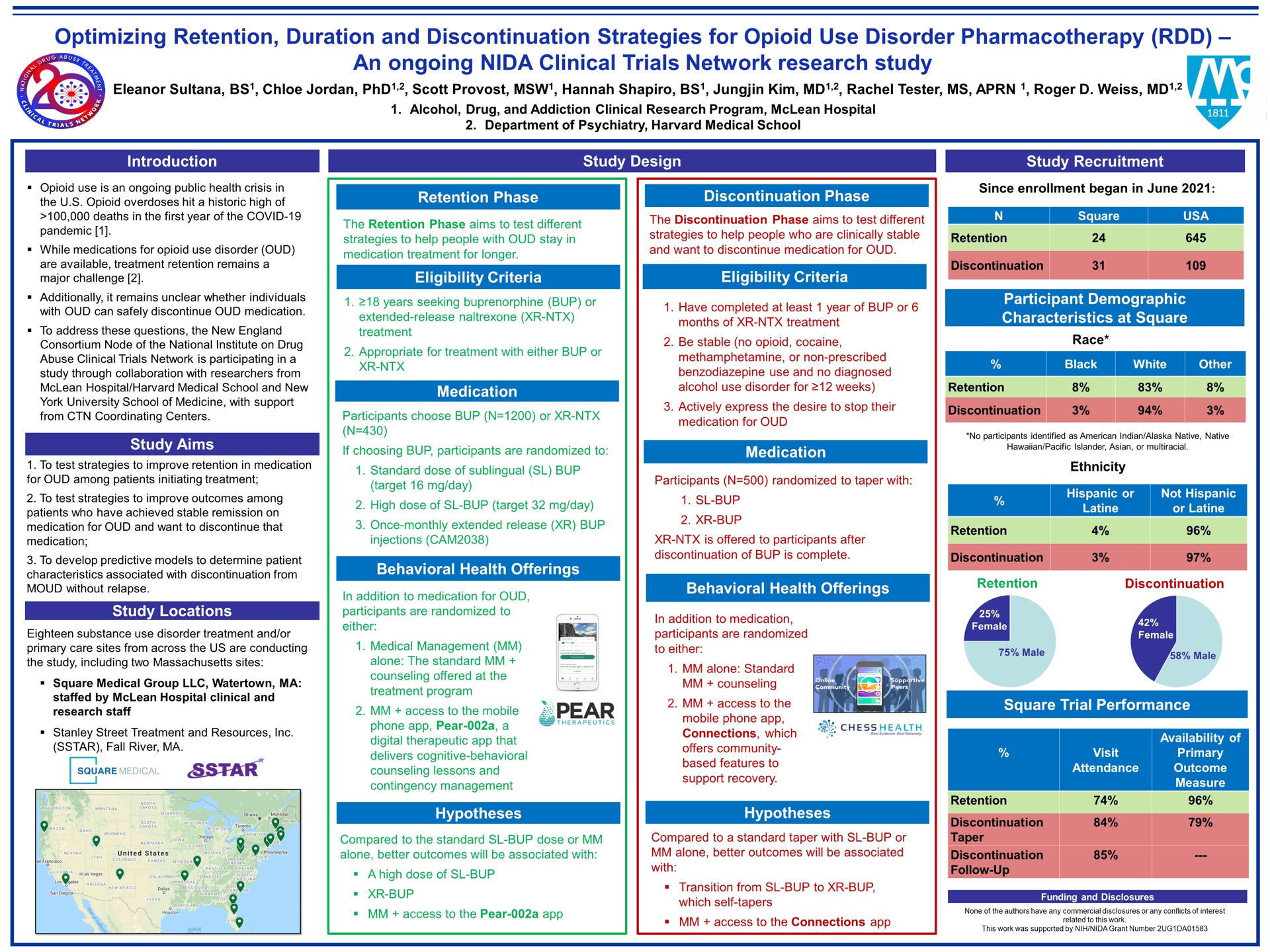
Background: Retention in medication treatment for opioid use disorder (MOUD) remains a major challenge. Moreover, for patients who are retained successfully on MOUD, do well, and then at some point want to stop MOUD, it is unclear how to advise patients about the likelihood that they will be able to safely discontinue MOUD without returning to opioid use. The NIDA Clinical Trials Network is conducting a study that aims to test: 1) strategies to improve retention in MOUD, 2) strategies to improve outcomes among stable patients who want to discontinueMOUD; and 3) predictive models to determine characteristics associated with successful MOUD discontinuation.
Methods: 18 sites are conducting this randomized, pragmatic, non-blinded study. The study consists of two phases. The Retention Phase, for patients entering a new treatment episode, is testing pharmacologic and behavioral strategies to improve retention. The Discontinuation Phase, for patients who are stable on MOUD and want to discontinue pharmacotherapy, is testing pharmacologic and behavioral strategies to see who can safely discontinue MOUD, and what modalities are optimal in doing so.
Retention Phase participants must be seeking MOUD and choose treatment with buprenorphine (BUP) [N = 1,200] or extended-release naltrexone (XR-NTX) [N = 430]. If choosing BUP, participants are randomized to 1) a standard dose of sublingual BUP (SL-BUP) t (target of 16 mg/day), 2) a high dose of SL-BUP (target of 32 mg/day) , or 3) a once-monthly extended-release injectable form of BUP (XR-BUP). Discontinuation Phase participants must have completed 1 year of BUP or 6 months of XR-NTX treatment, be stable (no opioid, cocaine, methamphetamine or non-prescribed benzodiazepine use and no diagnosed alcohol use disorder for ≥12 weeks), and actively express the desire to stop MOUD. Participants who have been taking SL-BUP before study entry are randomized to taper with SL-BUP or XR-BUP; those on XR-NTX require no taper from that medication [total N=500 SL-BUP; 250 XR-NTX]. In both phases, participants are also randomized to 1) standard Medical Management (MM), or 2) MM + access to technology-based digital therapeutic treatment: the Pear-002a app in the Retention Phase, which delivers cognitive-behavioral counseling and contingency management, or the Connections app in the Discontinuation Phase, which includes a menu of community-based features for recovery.
Hypothesized Results: In Retention, better outcomes are expected with the high SL-BUP dose and XR-BUP (each when compared to standard dose of SL-BUP), and with access to the Pear-002a app. In Discontinuation, better outcomes are expected with a transition to XR-BUP, which self-tapers, and with access to the Connections app.
Conclusions: Results from this study will inform future efforts to optimize OUD treatment retention outcomes and assist stable patients who are interested in discontinuing MOUD.
Search posters